Table of Contents
1. Purpose
2. General
3. Responsibilities
4. Procedures
5. Flowcharts
6. References
7. Attachments
1. Purpose
The objective of this procedure is to control all formal documents and data, that will be issued within Company.:
- Project deliverables and non-deliverables.
- Non-project related documents
2. General
This procedure describes responsibilities with respect to document control. How to prepare formal documents as described in ref. 6.2.
2.1 Definitions
|
Informal Document |
: |
An unauthorized document (e.g. notes for private use only, rough draft, in development). |
|
Formal Document |
: |
An authorized document. |
|
Controlled Document |
: |
A document which is controlled in accordance with this procedure (and clause 4.5 of ISO 9001:87) |
|
Quality System Document |
: |
A document that relates to the requirements of ISO 9001:87. |
|
Authorized |
: |
Confirmed by (e.g. originator, checker and approver). |
|
Review |
: |
Global verification to obtain confidence. |
|
Check |
: |
Detailed verification to obtain certainty. |
|
Approved |
: |
Authorized for issue (e.g. for information, for comments or released for design/purchase/ construction). |
|
Certified |
: |
The status of a document for which qualified engineers formally attest by signature, that the document fulfils the requirements. |
2.2 Document Grouping
All documents can be placed in one of the following groups:-
2.2.1 Informal Document
- distributed
- not-distributed
2.2.2 Formal Document
- Quality System Document:
a. Project Deliverable
b. Project Non-Deliverable
c. Non-Project Document
- Non-Quality System Document:
a. Project document
b. Non-Project Document
2.3 Document Control Requirements
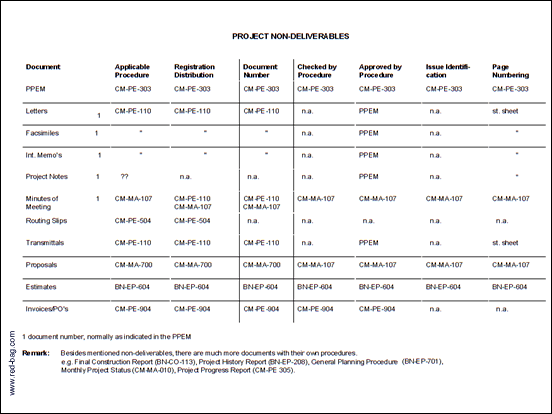
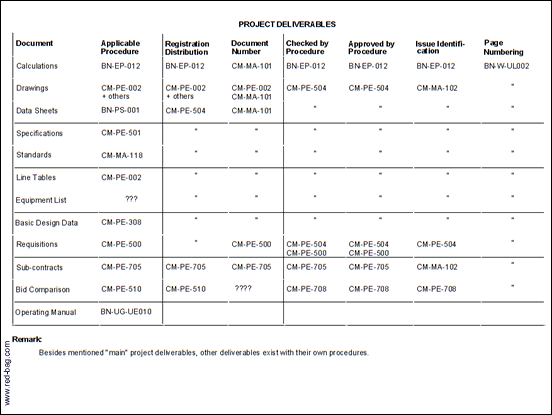
For many Quality System documents, procedures exist which describe specific requirements for preparation and/or registration, review, approval and distribution of these documents (Attachment 1).
Requirements mentioned in those procedures prevail above general requirements described in this procedure and ref. 6.2. It is the intention to remove all general document control requirements from those procedures which are covered in this procedure and ref. 6.2.
2.3.1 Informal Documents
All distributed informal documents shall have as a minimum:
- a document title/subject.
- a known origin/owner.
- a creation date.
- a page numbering.
No requirements exist for non-distributed informal documents.
Informal documents must be made formal, when there is a need to use them on projects (e.g. informally received client documents and E-mail).
2.3.2 Formal Documents
This procedure describes further only minimum requirements for formal documents!
All BBV formal documents shall:
- be registered to identify current revision.
- be authorized.
- have a unique document identification.
- have a clear issue status.
- be structured in accordance with ref. 6.2.
- have a known origin.
- have a traceable distribution.
- properly be filed to provide easy access.
- have an identification of changes, if appropriate.
Transmittals of "received/forwarded" formal documents shall be filed properly to enable traceability before distribution/use.
All Quality System Documents shall be reviewed and approved by authorized personnel prior to issue.
All Quality System Documents will be distributed in accordance with a predefined distribution schedule.
Obsolete issues will promptly be marked as obsolete or removed.
All project deliverables and non-deliverables shall be registered in a central document register to identify the current revision (e.g. IDCS).
All Project Deliverables shall be authorized in accordance with reference 6.3 and/or 6.4.
Changes on Project Deliverables shall be identified in the document or in a change list and reviewed by the same personnel, unless designated otherwise. Access to pertinent background information upon which to base their review and approval shall be provided by means of filing previous master documents and comments received.
3. Responsibilities
The Department or Project Manager is responsible for informing his personnel (verbal or in writing) of specific checking, approval, distribution and filing requirements for all formal documents.
The author is responsible for the document quality and providing background information, if appropriate.
The checker is responsible for review or checking (for engineering documents in general the lead engineer).
The approver is responsible for approval/certification, the distribution list and issue (description) of the document.
The document administrator will add the issue date and is responsible for maintaining the document register, reproduction, distribution and filing of the original document and filing all transmittals of "received/forwarded" documentation.
The recipient is responsible for filing the document and marking obsolete or removal of the superseded issue.
4. Procedures
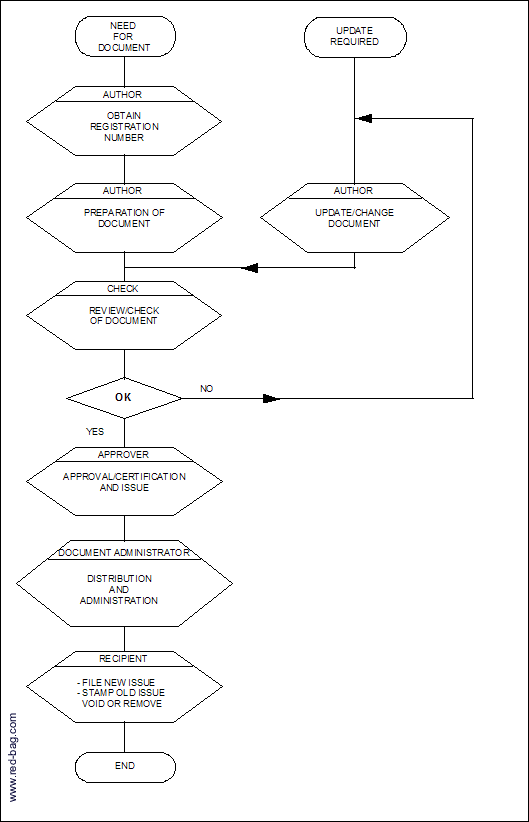
4.1 Procedure for the Preparation/Control of RB Documents
4.1.1 Registration
A document number (identification code) shall be obtained from the document administrator.
Many document numbers for the Quality System Documents are defined by:
|
References |
|||
|
Non Project Documents |
6.1 |
||
|
· Q.A. manual and related procedures |
|
||
|
· guides and standards |
|
||
|
Project Documents |
6.9 | ||
|
· project and specific procedures |
|
||
|
· guides, standards and specifications |
|||
|
· requisitions/purchase orders |
|||
|
· planning documents |
|||
|
· drawings/sketches/calculations |
|||
For other type of documents, the unique identification number may be prescribed by a specific procedure referenced in Attachment 1, the Project Procedure and Execution Manual or a departmental convention. The author must check this.
Every employee who expects a change in the number of project deliverables shall initiate an update of the document register, to enable proper progress measurement.
4.1.2 Preparation of the Document
All formal documents shall be written as such, that it is possible to check the completeness, validity and origin (see ref. 6.2):
- the document will clearly identify document number, issue code, date, description and authorization.
- the total number of pages is or can be established.
- every page shall bear beside a page number also the document number and issue code in case the document is not glued/bound.
- when a document will be (re)issued in parts, all parts shall be identified, with their valid issue (e.g. index system).
- all referred documents and/or documents which must be read in conjunction shall be identified, with their valid issue.
- the origin shall be identified (e.g. name of the author).
The formal documents shall be initialed by the author to signify he has completed and verified the quality of the document.
4.1.3 Authorization
All formal documents shall be reviewed and approved for adequacy by authorized personnel prior to issue (see also attachment 1).
Quality System Documents shall be authorized in accordance with the following references:
|
References |
|
|
Non Project Documents |
6.6 |
|
· QA Manual and related procedures |
6.6 |
|
· guides and standards |
6.6 |
|
· audit reports |
6.7 |
|
Project: |
|
|
· PPEM and related documents |
6.5 |
|
· engineering deliverables |
6.3 |
|
· process deliverables |
6.4 |
The use of these document shall be clear by the type and content of the document and/or the issue description.
4.1.4 Distribution and Administration
The organizations and persons to whom the document is to be distributed shall clearly be identified on the transmittal or accordingly to a controlled pre-defined distribution list.
In case an answer is requested, the due date shall clearly be identified on the transmittal and registered to enable chasing, if appropriate.
The document administrator shall handle only properly prepared and authorized documents (see 3.0 : "Responsibilities").
All issues of formal documents shall be registered prior to the distribution, indicating at a minimum the:
- document number and title.
- issue description, code and date.
e.g. the project document register shall contain all issues of project deliverables and non-deliverables, then the following shall be carried out:
- a copy of the document shall be filed.
- a copy of the transmittal and distribution list shall be filed consecutively in a "documents transmitted" file after check if the transmittal identifies the applicable documents with issue identification.
4.1.5 Receipt/Use
The recipient shall take care for proper filing (document structure must comply with new issued index, when the document is partly reissued) and removal of obsolete parts or stamp obsolete.
Quality system documents may only be used after verification of the validity with the document register.
4.1.6 Update/change
Anyone aware of a discrepancy relating to a (project) document shall discuss this with the originator, confirming by A.V.O. with a copy to all mentioned on the distribution list.
The author shall resolve all ambiguous or conflicting comments with the originators of these comments.
The author shall incorporate all valid comments in a new issue.
4.2 Procedure for Control of External Documents
4.2.1 Preparation (See Ref. 6.5)
During contract clarification and project kick-off meetings, the client shall be advised of detailed Company document control requirements. Any conflicting document control requirements between the Company and the client systems shall be resolved and properly described/referred to within the PPEM. In principal amendments to Company procedures will be written to instruct the task force.
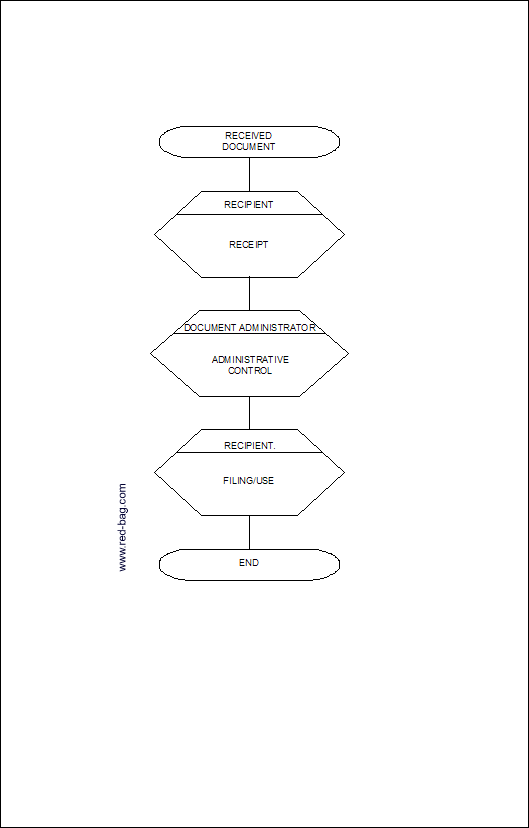
4.2.2 Receipt
Everyone in the organization who receives an external formal document, which is not yet registered, shall go promptly to the document administration to assure administrative control.
4.2.3 Administrative Control
Administrative control shall be maintained as follows:
- the document administrator shall register the document with a document received number (DR-).· a copy of the transmittal/distribution list shall be filed consecutively in a documents received file, after
- check if the transmittal identifies properly the received documents and issue identification.
- in case a reply is requested, the date shall be registered to enable chasing, if appropriate.
4.2.4 Filing and Use
The recipient shall take care of proper filing (document structure must comply with new issue index, when the document is partly reissued) and removal of obsolete parts or stamp obsolete.
In the case of project documents, one copy shall be filed in the central project file.
5. Flowcharts
5.1 Flowchart for Preparation/Control of BBV Documents
5.2 Flowchart for Control of Received Documents
6. References
|
Document Number |
Title |
Level |
|
|
6.1 |
BN-W-UL001 |
Numbering of Quality System Documents |
4 |
|
6.2 |
BN-W-U003 |
Preparation/Control of Formal Documents4 |
4 |
|
6.3 |
CM-PE-504 |
Production of Engineering Documents |
2 |
|
6.4 |
CM-PE-002 |
Preparation of Process Flow Diagrams, Engineering Flow Diagrams and Related Documents |
2 |
|
6.5 |
CM-PE-303 |
Project Procedure and Execution Manual |
2 |
|
6.6 |
CM-MA-100 |
Preparation of Quality System Documents |
2 |
|
6.7 |
CM-QA-001 |
Internal Quality and Safety Audits |
2 |
|
6.8 |
CM-PE-400 |
Verification, Approval and Transmittal of Process Engineering Documents |
2 |
|
6.9 |
BN-W-UE301 |
Numbering of Project Documents |
4 |
|
6.10 |
CM-PE-110 |
Procedure for Project File Coding and Maintenance |
2 |
| 6.11 |
CM-PE-101 |
Identification of Project References |
2 |
| 6.12 |
CM-PE-309 |
Compliance with Local Applicable Laws and Regulations |
2 |
|
6.13 |
CM-PE-310 |
Processing of Comments and Required Actions |
2 |
| 6.14 |
CM-PE-919 |
The Application of IDCS |
2 |
7. Attachments
7.1 List of Procedures with Specific Document Preparation/control Requirements (2 sheets)
Klick image for pdf file: